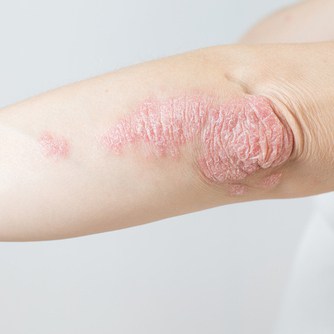
Risankizumab-rzaa (Skyrizi) is now approved for the treatment of adults with active psoriatic arthritis. It is the only IL-23 inhibitor approved for adults with moderate to severe plaque psoriasis and active psoriatic arthritis that can be administered with a single injection four times a year.
In approving the new indication, the FDA used data from two pivotal clinical trials that evaluated the efficacy and safety of risankizumab-rzaa in adults with active psoriatic arthritis, including those who had responded inadequately or were intolerant to biologic therapy and/or non-biologic disease-modifying antirheumatic drugs. The medication met the primary end points of both clinical trials at week 24, demonstrating significant improvement in joint symptoms, including swollen, tender, and painful joints compared with placebo. In addition, patients treated with risankizumab-rzaa in the trials showed improvement in dactylitis and enthesitis.
Risankizumab-rzaa is a product of AbbVie. Consult product labeling for full prescribing information.