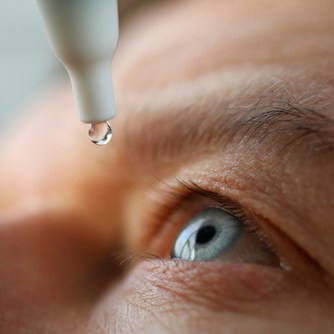
The FDA approved the first generic version of cyclosporine ophthalmic emulsion 0.05% single-use vials (Restasis) to increase tear production in patients whose tear production is presumed to be suppressed due to ocular inflammation associated with keratoconjunctivitis sicca. The new generic cyclosporine ophthalmic emulsion 0.05% is the first generic of the drug approved since the approval of Restasis in the United States nearly 20 years ago.
The approved generic of cyclosporine ophthalmic emulsion 0.05% is a product of Mylan Pharmaceuticals.
Editor’s note: this post was originally published on February 25, 2022 and updated on April 22, 2024.
Join our email list
Get new course alerts, newsletters and more delivered directly to your inbox
By providing my personal information, including phone number, I consent to (1) receive email messages with information and offers, autodialed calls, texts, and prerecorded messages from FHEA, including current and possible future services, customer service and billing; and (2) FHEA’s Privacy Policy and Terms and Conditions. I understand that my consent is not required to purchase, and that cancellation of purchase does not automatically revoke this consent.