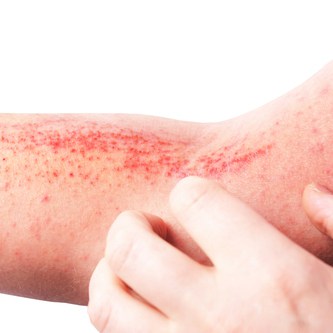
The FDA approved tralokinumab-ldrm (Adbry), a biologic for the treatment of moderate-to-severe atopic dermatitis in adults 18 years of age or older whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
The drug can be used with or without topical corticosteroids and is the first and only FDA-approved biologic that specifically binds to and inhibits IL-13 cytokine, a key driver of atopic dermatitis signs and symptoms. In clinical trials of tralokinumab-ldrm versus placebo, significantly more patients achieved primary and key secondary endpoint scores for clear or almost clear skin at week 16.
The most common adverse events reported in clinical trials of tralikinumab-ldrm were upper respiratory tract infections, conjunctivitis, injection-site reactions, and eosinophilia.
Tralokinumab-ldrm is a product of Leo Pharma and is available in a 150 mg/mL prefilled syringe for subcutaneous injection with an initial dose of 600 mg followed by 300 mg every other week.
Consult product labeling for detailed prescribing information.