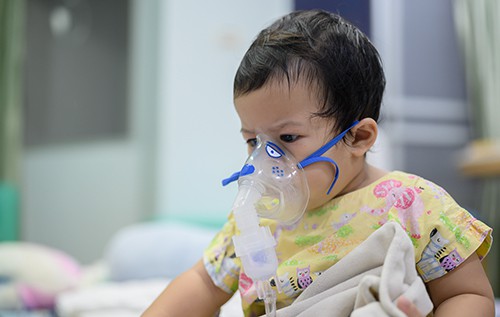
U.S. Food and Drug Administration approved Beyfortus (nirsevimab-alip) for the prevention of Respiratory Syncytial Virus (RSV) lower respiratory tract disease in infants during their first RSV season. It is also approved for children up to two years old vulnerable to severe RSV through their second RSV season.
Recommended course for NPs: Hot Topics: The Surge: What Clinicians Need to know about RSV
While most infants experience mild, cold-like symptoms when infected with RSV, some may develop lower respiratory tract disease such as pneumonia and bronchiolitis.
Approximately 1% to 3% of children under 12 months of age in the United States are hospitalized each year due to RSV, according to the American Academy of Pediatrics. Premature infants, and those with chronic lung disease of prematurity or significant congenital heart disease, are at highest risk for severe RSV disease.
“RSV can cause serious disease in infants and some children and results in a large number of emergency department and physician office visits each year,” said John Farley, M.D., M.P.H., director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research. “Today’s approval addresses the great need for products to help reduce the impact of RSV disease on children, families and the health care system.”
According to the FDA, Beyfortus is a monoclonal antibody with activity against RSV. The drug’s safety and efficacy were supported by three clinical trials (Trials 03, 04 and 05). The key measure of efficacy was the incidence of medically attended RSV lower respiratory tract infection (MA RSV LRTI), evaluated during the 150 days after Beyfortus administration.
Read the full press release here.