-

First Vaccine for Pregnant Individuals to Prevent RSV in Infants Approved by FDA
On August 21, 2023, the U.S. FDA approved Abrysvo, a Respiratory Syncytial Virus vaccine, for pregnant individuals. The…
-

FDA Approves First Over-the-Counter Birth Control Pill in the U.S.
On July 13, 2023, the FDA approved Opill (norgestrel) tablet for nonprescription use to prevent pregnancy. Opill is…
-
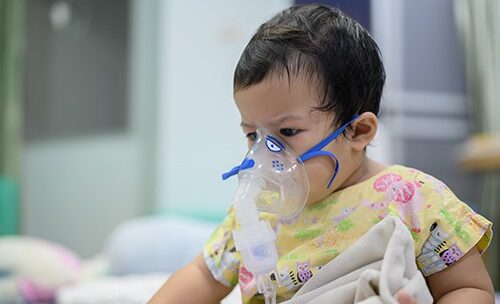
New Drug to Prevent RSV in Infants and Toddlers Approved By FDA
U.S. Food and Drug Administration approved Beyfortus (nirsevimab-alip) for the prevention of Respiratory Syncytial Virus (RSV) lower respiratory…
-

First Drug to Treat Agitation Symptoms Associated with Alzheimer’s-Related Dementia Approved by FDA
On May 11th, the FDA announced its supplemental approval of Rexulti (brexpiprazole) oral tablets for treatment of agitation…
-
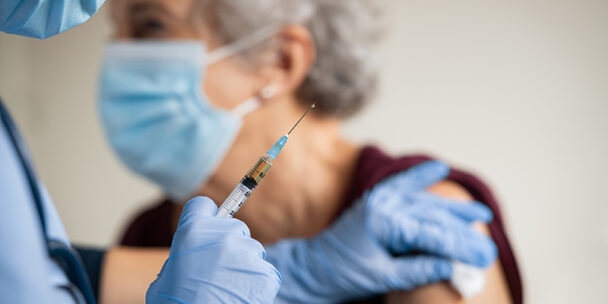
First Respiratory Syncytial Virus (RSV) Vaccine for Adults 60 Years and Older Receives FDA Approval
On May 3rd, the FDA approved Arexvy, the first RSV vaccine available in the United States for individuals…
-
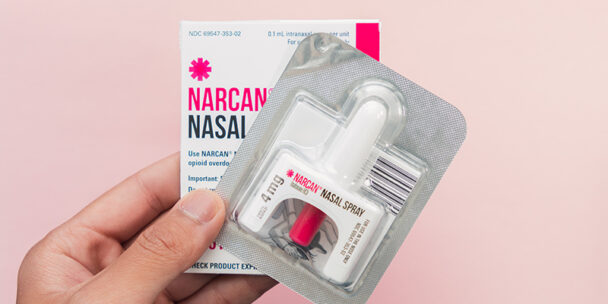
FDA Approves Naloxone Nasal Spray for Nonprescription Use
The U.S. Food and Drug Administration approved Narcan, 4 milligram (mg) naloxone hydrochloride nasal spray, for over-the-counter nonprescription…
-
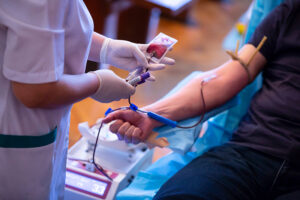
FDA Releases Draft Revisions to Blood Donor Eligibility Evaluations
The FDA has released draft guidelines revising recommendations for evaluating blood donor eligibility. The proposed evaluative guidelines will…
-
CDC and FDA Tracking Outbreak of Drug-Resistant Pseudomonas aeruginosa
The CDC and FDA are investigating the outbreak of VIM-GES-CRPA, a rare strain of extensively drug-resistant P. aeruginosa. …
-

FDA Approves New Drug for Early Alzheimer’s Treatment
Early this year, the FDA approved Alzheimer’s treatment drug Leqembi (lecanemab-irmb) using its Accelerated Approval pathway. Accelerated Approval…
-

Topical Medication for Plaque Psoriasis in Adults Approved by FDA
The FDA approved tapinarof cream 1% (VTAMA), a steroid-free topical cream applied once daily for the treatment of…
-

FDA Approves Two Products for H pylori Infection
The FDA approved vonoprazan, amoxicillin, and clarithromycin (Voquezna Triple Pack) and vonoprazan, amoxicillin (Voquezna Dual Pak) for the…
-

Drug Approved for Recurrent Vulvovaginal Candidiasis
Oteseconazole (Vivjoa) was approved by the FDA to reduce the incidence of recurrent vulvovaginal candidiasis (RVVC) in females…
Trending
Join our email list
Get new course alerts, newsletters and more delivered directly to your inbox
By providing my personal information, including phone number, I consent to (1) receive email messages with information and offers, autodialed calls, texts, and prerecorded messages from FHEA, including current and possible future services, customer service and billing; and (2) FHEA’s Privacy Policy and Terms and Conditions. I understand that my consent is not required to purchase, and that cancellation of purchase does not automatically revoke this consent.